Atomic Spectrum (Hydrogen Spectrum)
Atomic Spectrum (Hydrogen Spectrum): Overview
This topic covers concepts, such as, Hydrogen Emission Spectrum, Explanation of Line Spectrum of Hydrogen, Calculation of Number of Spectral Lines & Interpretation of Hydrogen Spectrum etc.
Important Questions on Atomic Spectrum (Hydrogen Spectrum)
The frequency of oscillation of the electric field vector of a certain electromagnetic.wave is Hz. What is the frequency of oscillation of the corresponding magnetic field vector and to which part of the electromagnetic spectrum does it belong?
The spectrum of He is expected to be similar to that
According to Bohr theory, the electronic energy of a hydrogen atom in the Bohr atom is given by . Calculate the longest wavelength of light that will be needed to remove and electron from the third Bohr orbit of the ion (If the wavelength is (in meter) and is an integer. Report '')
In the Balmer series of atomic spectra of hydrogen, there is spectral line having wavelength . Calculate the number of higher orbit from which the electron drops to generate this line.
If the series limit to the wavelength of the Lyman series of the hydrogen atom is , then the series limit to the wavelength for the Balmer series of the hydrogen atom is
Assertion : The spectrum of is expected to be similar to that of hydrogen.
Reason : is also one electron system.
Which of the following is the value of the Rydberg constant for hydrogen?
Among the following, which transition in the Hydrogen spectrum would have the same wavelength as Balmer transition, to in spectrum?
If the wavelength of the first line in Balmer series is , then the wavelengths of its second line and limiting line respectively are
Which energy level transition among the following will have the least wavelength?
The electron in a hydrogen atom jump on absorbing of energy would jump to orbit
The shortest wavelength in hydrogen spectrum is approximately ______
Which of the following series of transitions in the spectrum of hydrogen atom falls in visible region?
Calculate the shell number of excite state of -atom in terms of wavelength , when the electron excites to the ground level.
In the Balmer series of atomic spectra of hydrogen, there is spectral line having wavelength . Calculate the number of higher orbit from which the electron drops to generate this line.
What transition in the spectrum would have the same wavelength as the first Lyman transition of hydrogen ?
The frequency of the spectral line when an electron from the fifth orbit jumps to the second orbit in a hydrogen atom is . Calculate the value of x?
The frequency of radiation having wavelength is . The value of is
Roundoff the answer up to one significant figures.
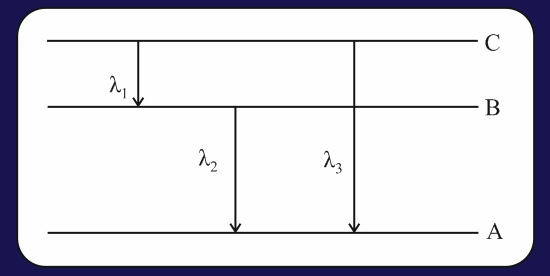
Energy levels of of a certain atom correspond to the increasing value of energy, i.e.. If
Suppose that an excited hydrogen atom returns to the ground state, the wavelength of the emitted photon is . What will be the principal quantum number of the excited state?
